In the realm of scientific revelation, certain experiments mark pivotal moments, altering the very foundation of our understanding. One such landmark series, known as the Geiger-Marsden experiments or the Rutherford gold foil experiment, unmasked an elemental truth about atoms that had eluded comprehension for centuries. While the concept of atoms had lingered in ancient civilizations, the 20th century’s scientific strides sought to demystify their true nature.
However, the unraveling of fundamental concepts often hinges on our willingness to embrace novel perspectives and leverage emerging technologies and mathematical frameworks. This struggle to shift entrenched views complicates the comprehension of rudimentary principles, making revelations like those from the Geiger-Marsden experiments all the more crucial.
Why was gold used in Rutherford’s experiments to discover an atom?
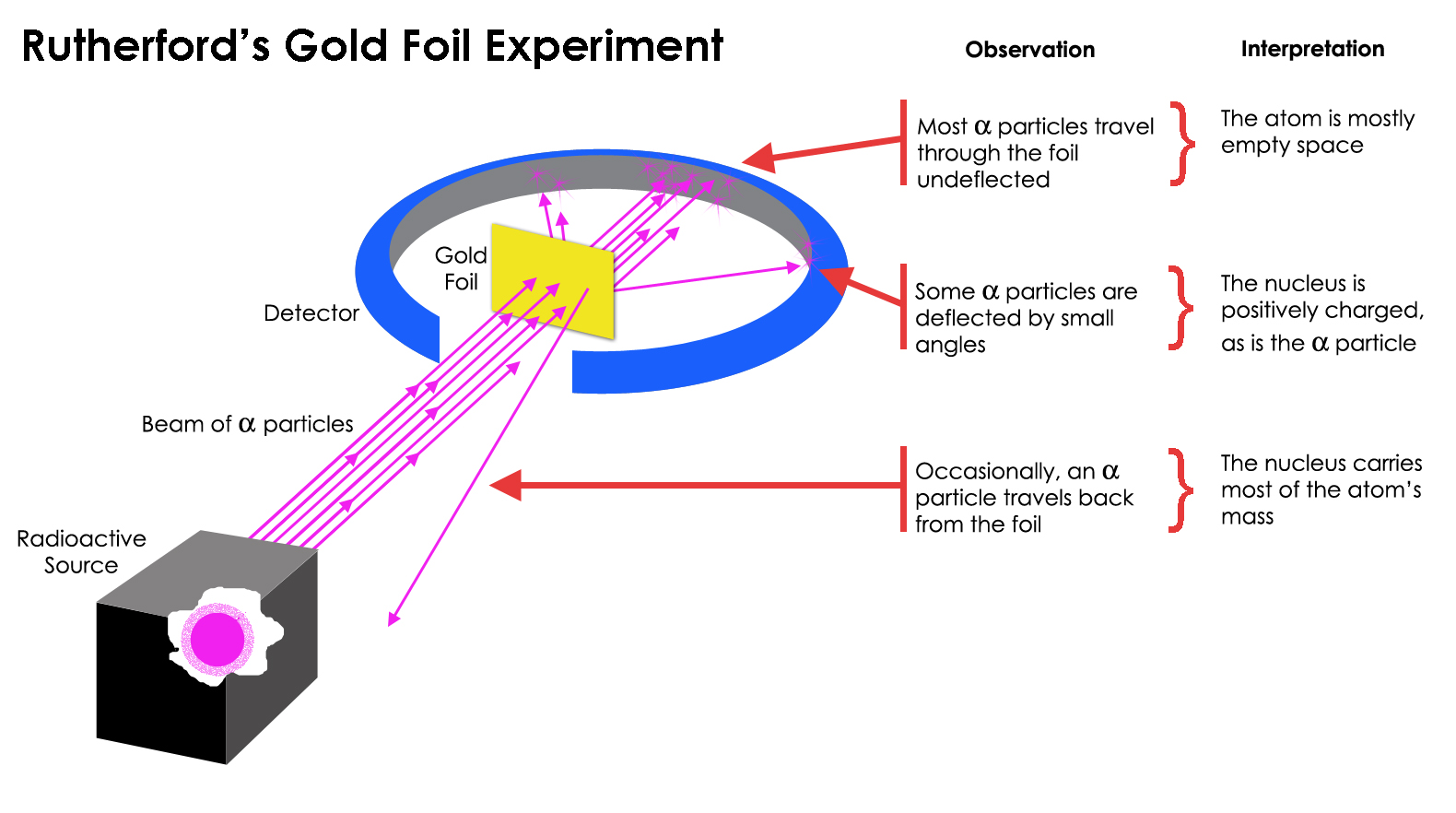
Rutherford used gold in his famous experiment because of its malleability. He wanted a thin enough layer of gold that alpha particles (positively charged particles) could pass through it. By using a thin gold foil, he aimed to observe the behavior of these alpha particles when they interacted with the atoms in the foil.
This helped him understand the structure of the atom and led to the discovery of the atomic nucleus. The surprising result was that while most of the alpha particles passed through the foil, some were deflected at large angles or even bounced back, suggesting that the majority of an atom’s mass and positive charge were concentrated in a small, dense region – the nucleus.
Exploration and Findings
Ernest Rutherford, a key figure in this narrative, orchestrated a sequence of experiments at the University of Manchester, collaborating with Hans Geiger and Ernest Marsden. Their objective: to delve into the inner workings of atoms, challenging prevalent notions and theories:
- The experiment’s essence lay in sending alpha particles through materials, particularly gold foil, anticipating slight deflections if Thomson’s plum pudding model, portraying the atom as a positively charged sphere with scattered electrons, held true;
- Astonishingly, the results revealed not mere deflections but drastic deviations, with some particles rebounding, akin to firing at a target only to have the bullet ricochet back.
This unexpected phenomenon upended conventional wisdom. Rutherford deduced the presence of a concentrated, positively charged nucleus within the atom, shattering existing perceptions and marking a revolutionary juncture in atomic understanding.
What would have happened if Rutherford used copper instead of gold?
If Rutherford had used copper instead of gold in his experiment, the results might have been similar in principle but with some differences. Copper, like gold, is malleable and could have been made into a thin foil for the experiment. However, the key aspects of the experiment’s outcome might have varied due to the different atomic properties of copper compared to gold.
- Since the experiment aimed to understand the structure of atoms by bombarding them with alpha particles, the behavior of these particles when interacting with the atoms in the foil would have been observed. The crucial discovery was the unexpected deflection of alpha particles, indicating a concentrated positive charge and mass at the center of the atom, later understood as the atomic nucleus;
- Gold was chosen because it was highly malleable and could be made into very thin foils, allowing alpha particles to pass through. The choice of gold was not due to specific properties of gold atoms but rather the suitability of gold foils for the experiment. Copper, while also malleable, might have produced slightly different results due to variations in its atomic structure and density compared to gold. The concentration of mass and positive charge in the atomic nucleus might still have been observed, but the degree of deflection and the precise outcomes could have differed.
In essence, the fundamental conclusions about the structure of the atom might not have changed drastically, but the specifics of the experimental results might have been altered if a different material, like copper, was used.
Significance of Gold
The choice of gold within the experiments was strategic. Observations indicated that heavier elements, like gold, exhibited greater reflection of alpha particles compared to lighter metals. Geiger’s exploration further illuminated gold’s suitability: its malleability allowed for ultra-thin foils, crucial for unimpeded particle transmission during experimentation.
Rutherford’s revelation prompted a paradigm shift in atomic models. While his model initially defied Newtonian mechanics, the subsequent integration of quantum mechanics birthed the Rutherford-Bohr model. This quantum interpretation revised the notion of electron orbits, unveiling a probabilistic cloud-like structure around the nucleus.
Gold held a pivotal role in Rutherford’s experiments due to several crucial factors:
- Reflective Properties: Gold’s atomic structure and density made it an ideal candidate for the experiments. Compared to lighter metals like aluminum, gold demonstrated a higher capability to reflect alpha particles. This property allowed for clearer observation and measurement of particle deflections, providing tangible evidence that challenged prevailing atomic models;
- Malleability: Gold’s exceptional malleability enabled the creation of ultra-thin foils, almost as thin as a single atom’s thickness. This thinness was imperative for the experiment’s success, ensuring that alpha particles could pass through the material relatively unimpeded. The ability to create such thin foils was crucial in allowing particles to interact with the gold atoms and showcase the unexpected behavior observed in the experiments;
- Consistency and Purity: Gold’s consistent atomic structure and high purity were advantageous for experimental precision. The uniformity of gold foils ensured a standardized medium for the alpha particles’ transmission, reducing potential confounding variables and enhancing the reliability of the results.
- Experimental Viability: The practicality of working with gold, both in terms of availability and workability in the laboratory, made it a pragmatic choice for these critical experiments. Its properties aligned perfectly with the experimental requirements, allowing for repeated, controlled trials that yielded consistent and enlightening results;
- Scientific Impact: The unique behavior observed with gold in these experiments, where some alpha particles were reflected at large angles or even back towards the source, directly challenged prevailing atomic theories. This unexpected outcome led Rutherford to propose the existence of a concentrated, positively charged nucleus within atoms, fundamentally altering our understanding of atomic structure and setting the stage for subsequent developments in nuclear physics.
In essence, gold’s reflective properties, malleability, consistency, and suitability for controlled experimentation played a pivotal role in facilitating the observations that unveiled the existence of the atomic nucleus, forever altering the landscape of atomic theory.
Conclusion
The Rutherford gold foil experiment stands as a cornerstone in scientific history, a testament to the potency of experimentation and the readiness to challenge established doctrines. Its legacy endures, illuminating the iterative nature of scientific discovery and the transformative power of probing the fundamental fabric of our universe.